What Questions Should I Ask My Doctor
If your baby has hypoplastic left heart syndrome, you may want to ask your healthcare provider:
- What are the risks of surgery?
- Are there potential surgery complications to watch for?
- What medications does my child need?
- Are there any medication side effects?
- How will HLHS affect my childs life?
- What follow-up care does my child need after surgery?
- If I have another baby, does that child have a higher risk of HLHS?
A note from Cleveland Clinic
Having a child with hypoplastic left heart syndrome can be stressful and lonely. Its important to find a support group or other source of emotional support while you deal with the stresses and uncertainties of your childs heart defect. Feeling emotionally strong will help you manage the highs and lows of your childs health.
Last reviewed by a Cleveland Clinic medical professional on 07/29/2022.
References
Treatment Option : Heart Transplant
Heart transplant involves replacing a sick heart with a new heart . There are risks involved with heart transplantation. Children will need to be on lifelong medications to prevent their own body from attacking the heart which is called rejection. Long-term complications of transplant include potential rejection, infection, coronary disease, and a special type of lymphoma associated with immunosuppression. Children who require the transplant will be placed on a waiting list administered by a national agency called United Network for Organ Sharing. The wait time can be days, months or even more than a year. Some babies may pass away while on the waiting list. A transplanted heart will last an average of 8-14 years. Some children also require repeat transplantation.
Worldwide Leaders In Hypoplastic Left Heart Syndrome Surgery Survival Rates
Herma Heart Institute specializes in repairing Hypoplastic left heart syndrome and single ventricle defects, and has the best published HLHS survival rates worldwide. Our institute consistently outperforms when it comes to congenital heart surgery outcomes for even the most complex types of heart disease, as evaluated by the Society of Thoracic Surgeons. Treatment for HLHS requires our highly specialized congenital heart surgeons to rework the baby’s circulatory system through a series of three open heart surgeries staged over a span of about three years. An alternative approach is heart transplantation, also an area of expertise at Children’s Wisconsin. See survival rate outcomes specific to the Norwood, Glenn and Fontan procedures.
Don’t Miss: How To Control Heart Rate
Hypoplastic Left Heart Syndrome
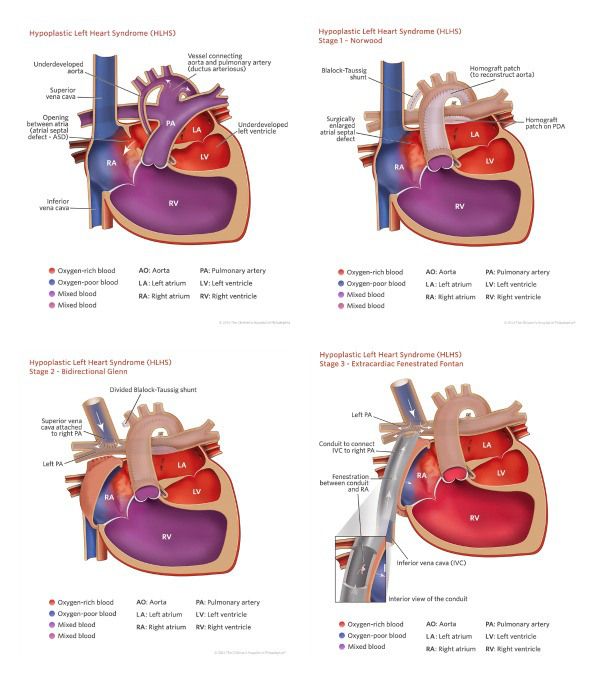
In hypoplastic left heart syndrome, the left side of the heart – the part that pumps oxygenated blood to the rest of the body – is underdeveloped. Its two chambers, called the left atrium and the left ventricle, and their valves may be tiny, blocking the flow of oxygenated blood from the lungs.
The heart consists of four chambers: the two upper chambers, called atria, where blood enters the heart, and the two lower chambers, called ventricles, where blood is pumped out of the heart. The flow between the chambers is controlled by a set of valves that act as one-way doors.
Normally blood is pumped from the right side of the heart through the pulmonary valve and the pulmonary artery to the lungs, where the blood is filled with oxygen. From the lungs, the blood travels back down to the left atrium and left ventricle. The newly oxygenated blood then is pumped through another big blood vessel called the aorta to the rest of the body.
Babies with hypoplastic left heart syndrome may seem healthy at birth because the patent ductus arteriosus is still open. The PDA is a blood vessel that connects the pulmonary artery to the aorta, allowing blood to continue circulating directly into the aorta and out to the rest of the body, bypassing the lungs and the defective left side of the heart. Once the PDA closes a few days after birth, blood flows to the lungs and then to the left side of the heart, where it is blocked and can’t circulate through the rest of the body.
Infants Who Have These Surgeries Are Not Cured
Infants with hypoplastic left heart syndrome may have lifelong complications. They will need regular follow-up visits with a cardiologist to monitor their progress. If the hypoplastic left heart syndrome defect is very complex, or the heart becomes weak after the surgeries, a heart transplant may be needed. Infants who receive a heart transplant will need to take medicines for the rest of their lives to prevent their body from rejecting the new heart.
You May Like: At What Heart Rate Should I Go To The Hospital
Description Of The Surgical Technique
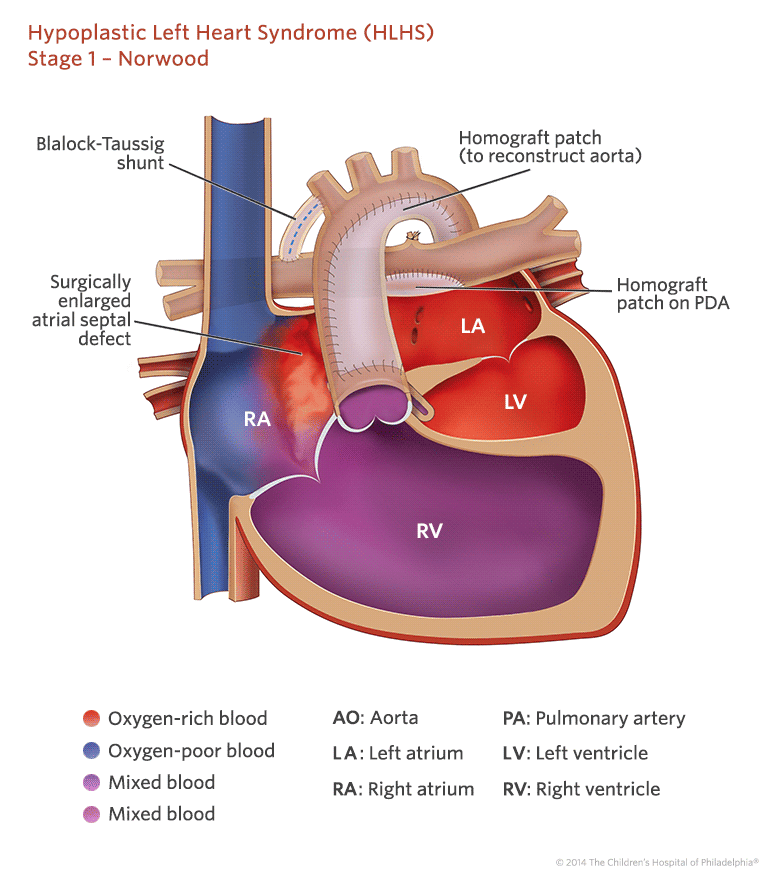
Stage 1
Surgical hybrid strategy for HLHS: Under CPB and a beating heart, and after atrial septal defect enlargement via right atriotomy, the PDA is replaced by a T-shaped pulmonary homograft while the left and right PA are banded.
Under CPB with bicaval cannulation and arterial cannulation in the innominate artery, the interatrial septum is resected via right atriotomy. The patent ductus arteriosus is replaced by a T-shaped pulmonary homograft while bilateral PA banding is performed using a GoreTex suture that is tight on a Hegar dilator.
Interstage
After discharge, the patients were closely followed: echocardiographic assessment was done 1 month after discharge and before Stage 2 cardiac catheterization.
Echographic assessment was used to monitor blood flow in the pulmonary homograft, evolution of the atretic ascending aorta, placement and gradient of the PA bands, right ventricular function, and analysis of the tricuspid valve. Whenever the results of this interstage assessment were abnormal or if the patient was symptomatic, the patient was examined with either computed tomography or angiography.
Pre-stage 2 cardiac catheterization was used to evaluate pulmonary vascular resistance and to determine whether it was safe to proceed with Stage 2, to analyse the systemic blood flow in the pulmonary homograft, the ascending and the descending aorta and to detect any PA distortion due to PA banding.
The patients were scheduled for Stage 2 when they were 46 months of age.
Differential Expression Of Mirnas In Pediatric Hlhs Patients
miRNA expression in HLHS patients. Expression profile of miRNAs in non-failing HLHS pediatric patients by miRNA array. Green down-regulated Red up-regulated. Only miRNAs with a qvalue of < 0.025 are shown. miRNAs are presented by increasing qvalue. N=6 non-failing right ventricle , 10 post-stage 1, 5 post-stage 3 HLHS patients. Supervised hierarchical clustering of array samples based on Ward’s method. Relative expression of a subset of miRNAs in non-failing, post-stage 1 and post-stage 3 HLHS patients was confirmed by RT-PCR, as described in the Methods section. N=9 non-failing, 16 post-stage 1, 6 post-stage 3 HLHS patients. Statistically significant expression of miRNAs is shown in the Figure.
You May Like: Laparoscopic Bypass Surgery Heart
Comparison With Classic Norwood
We compared the results of our new surgical approach with our poor results using the classic Norwood procedure for neonates with HLHS. We found significant differences in CPB and selective perfusion times , need for delayed chest closure and in-hospital deaths after surgery , in favour of the new surgical hybrid approach . Death before Stage 2 and interstage deaths were lower with the new surgical hybrid procedure than with the classic Norwood procedure . Although not significant, postoperative complications such as the need for extracorporeal membrane oxygenation, mechanical ventilation, inotropic support, or peritoneal dialysis was higher with the classic hybrid Norwood procedure. Median stays in the ICU and hospital decreased by 6 and 4 days, respectively, with the new surgical hybrid procedure.
Third Stage Of Treatmentfontan Procedure
The Fontan operation typically connects the IVC to the right pulmonary artery leading to a total cavopulmonary connection so that all PBF is achieved passively. In-series circulation is restored and saturation achieves near-normal levels. This typically happens between 24 and 48 months of age. There are numerous single institution series investigating the short- and long-term outcomes and predictors of mortality and morbidity after the Fontan completion . The consistent predictors of poor outcome across multiple studies are longer cross-clamp times, longer duration of hospital stay, heterotaxy, and atrioventricular valve anomaly.
Total venous-pulmonary connection may also be done by intervention cardiology via a polytetrafluorethylene-covered stent . Balloon expansion under the control of angiography and echocardiography allows for an accurate placement of the stent in the correct position. This procedure requires prior preparation in II stage of treatment. The advantage is it may be done without ECMO support.
You May Like: Congestive Heart Disease
Keeping The Duct Patent
The ductus arteriosus can be kept patent via the use of prostaglandin E1 . The previously described starting dose range of 0.050.10 mcg/kg/min has fallen out of favor to a lower starting dose of 0.01 mcg/kg/min . It is most probable that these previously higher doses were the starting doses for patients who had not been prenatally diagnosed and who came to medical attention later in the neonatal period. The risk of precipitating apnea is higher at higher starting doses. The recommended maintenance dose of PGE is 0.010.04 mcg/kg/min. At our institution, we will use doses as low as 0.003 mcg/kg/min0.005 mcg/kg/min while patients await surgical repair. There are still institutions that will maintain their patients on PGE1 for prolonged periods of time.
The other means by which the ductus arteriosus can be maintained patent is through stenting of the ductus arteriosus. For patients with HLHS, this is generally done as part of the Hybrid procedure.
What Tests Will Be Done To Diagnose Hypoplastic Left Heart Syndrome
Tests for a hypoplastic left heart syndrome diagnosis may include:
- Chest X-ray: This shows the size and shape of your babys heart and lungs.
- Echocardiogram: This ultrasound shows internal heart structures.
- Electrocardiogram : This measures electrical changes during a heartbeat.
- Pulse oximetry screening: This tells how much oxygen is in your babys bloodstream.
Read Also: How Do They Do Open Heart Surgery
What Is The Survival Rate Of Hypoplastic Left Heart Syndrome
About 20% to 60% of babies with hypoplastic left heart syndrome survive their first year of life. After that, the survival rate for the next five, 10 and 15 years is about 40%. Babies who have a normal birth weight and arent premature do better than babies with lower birth weights. One study found that most babies who survived their first year were still alive at age 18.
Why Choose Norton Childrens Heart Institute
- Norton Childrens Hospital has been a pioneer in pediatric cardiothoracic surgery, performing Kentuckys first pediatric heart transplant in 1986 and becoming the second site in the United States to perform an infant heart transplant.
- The American Board of Thoracic Surgery has certified our cardiothoracic surgeons in congenital heart surgery.
- The Adult Congenital Heart Association has accredited Norton Childrens Heart Institutes Adult Congenital Heart Program as the only comprehensive care center in Kentucky and Indiana treating adults born with a heart defect.
- More than 5,000 children a year visit Norton Childrens Heart Institute for advanced heart care.
- Norton Childrens Heart Institute has offices across Kentucky and Southern Indiana to bring quality pediatric heart care closer to home.
- The Jennifer Lawrence Cardiac Intensive Care Unit at Norton Childrens Hospital is the largest dedicated CICU in Kentucky, equipped with 17 private rooms and the newest technology available for heart care.
Read Also: Is 116 Heart Rate High
Symptoms Of Hypoplastic Left Heart Syndrome
Most babies with HLHS develop symptoms within the first days after birth if they do not get the medical care they need.
Common symptoms include:
- Blue or purple-tinged skin, lips or fingernails or skin that looks mottled, grayish or paler than your babys usual skin color
- Being more tired than is normal
- Trouble feeding
Treatment Option : Hybrid Approach
The Hybrid procedure is done in the Hybrid Suite. This specialized suite is a combined operating room and cardiac catheterization room. The child does not require heart-lung bypass for the first stage. It can be used on smaller weight infants and for families who request Alternative Non-Blood Medical Management. Stage 1 usually is done within the first week of life.
You May Like: How To Reduce Your Heart Rate
After The Baby Is Born
Babies with hypoplastic left heart syndrome might not have trouble for the first few days of life while the patent ductus arteriosus and the patent foramen ovale are open, but quickly develop signs after these openings are closed, including:
- Problems breathing,
- Ashen or bluish skin color.
During a physical examination, a doctor can see these signs or might hear a heart murmur . If a murmur is heard or other signs are present, the health care provider might request one or more tests to make a diagnosis, the most common being an echocardiogram. Echocardiography also is useful for helping the health care provider follow the childs health over time.
HLHS is a defect that also can be detected with newborn pulse oximetry screening. Pulse oximetry is a simple bedside test to determine the amount of oxygen in a babys blood. Low levels of oxygen in the blood can be a sign of a CCHD. Newborn screening using pulse oximetry can identify some infants with a CCHD, like HLHS, before they show any symptoms.
The Hybrid Stage I Consists Of:
Placement of bands around the right and left pulmonary arteries. This restricts blood flow to the pulmonary arteries, thereby balancing the circulation . This also reduces the extra blood flow to the lungs.
Stenting of the PDA keeps the PDA open and maintains the connection to the aorta and the body’s circulation.
There must be enough of a connection between the top two chambers of the heart to provide open blood flow and mixing of the oxygen-rich and oxygen-poor blood. This can be done through a balloon atrial septostomy. In this procedure, a small tube is passed across the septum between the two atria. Then a balloon on the end of the catheter is inflated and pulled back across the septum to enlarge the opening.
The goals of this first surgery stage include:
Open blood flow through the PDA,
An unblocked atrial septum
Balanced blood flow through the lungs and rest of the body.
Also Check: Does Stress Increase Heart Rate
Is Hypoplastic Left Heart Syndrome Treatable
Yes. First, your baby will need a medication called prostaglandin. This keeps their ductus arteriosus open and functioning. Other medicines can help your babys heart work more efficiently. Your baby also may need help with breathing.
Then theyll need a series of surgeries to direct blood flow to their lungs and body. These operations put their hearts workload on their right ventricle, which does all the pumping.
How Do I Take Care Of My Child
Children born with this condition can live a healthy life with long-term monitoring from a cardiologist.
You can care for your child in these ways:
- Make sure your child gets vaccinated against the flu every year and against COVID-19.
- Bring your child to cardiologist appointments every six months or every year.
- Make sure your child takes their medications.
- Limit your childs intense physical activity based on their providers recommendations.
- Work with them if they have learning difficulties.
Read Also: What Causes Coronary Heart Disease
Treatment For Hypoplastic Left Heart Syndrome
Hypoplastic left heart syndrome is most often fatal without early intervention. Compared to 25 years ago, there are now many different options for treatment of this complex heart condition an individualized approach is taken for each and every child. Your doctor will explain each individual option, and why one particular approach might be recommended for your child.
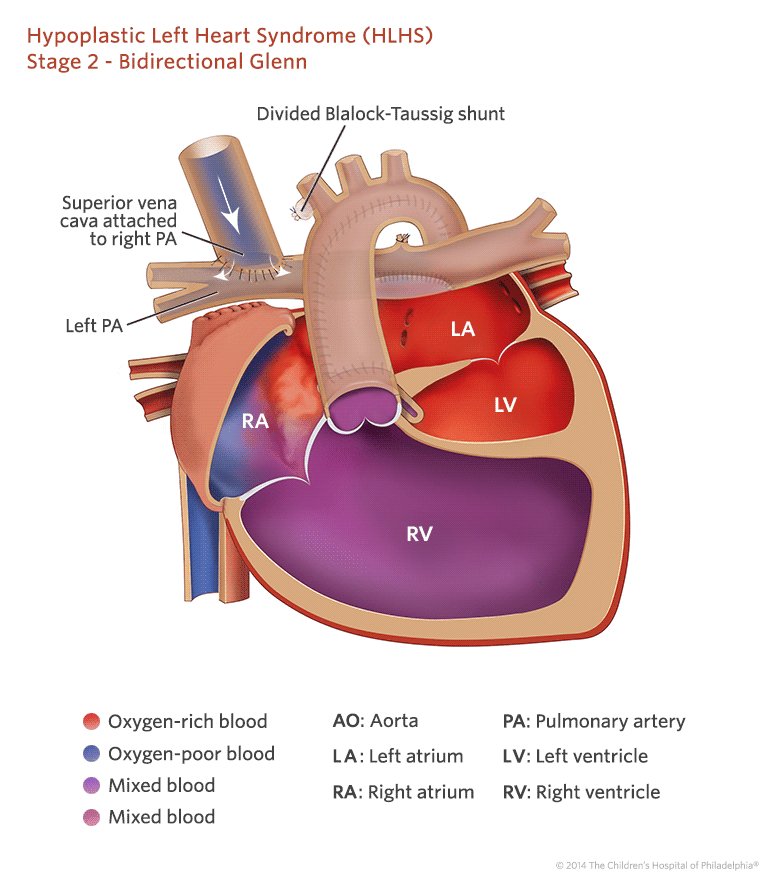
Stage Ii: Glenn Shunt
For this procedure, the baby is again put on the heart-lung bypass machine. The superior vena cava is attached directly to the pulmonary artery and the BT shunt is removed. This cardiac surgery is less involved than the Norwood procedure because so much of the work was done when the child was a newborn. This procedure is usually done around 6 months of age.
You May Like: Heart Valve Replacement Surgery Cost In India
Future Of Stem Cell Therapies
A systematic review found 23 articles, published since 2010, as well as nine relevant clinical trials related to congenital heart disease and recent advances in stem cell therapies. Pre-clinical research has focused on several types of stem cells including: mesenchymal stem cells , autologous umbilical cord blood cells, c-kit+ cardiac stem cells, and neonatal thymus mesenchymal stem cells. These cell types have shown the ability to differentiate into cardiac tissue making them ideal for cardiac regenerative therapy. The first use of autologous umbilical cord blood cells was done at the Mayo Clinic in 2015 and was found to increase right ventricular function in the patient after their procedure. The first use of cardiac progenitor cells occurred in the Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology Trail of 2011.
Home Monitoring Between Stages 1 And 2
During the period between stage 1 and stage 2 surgeries, your babys heart circulation requires extra monitoring to prevent complications. Our Home Monitoring Program will give you goals for your babys growth and oxygen saturation levels and provide monitoring equipment for your baby. Youll also get information on when to call.
Also Check: What Are Some Heart Disease
Second Stage Of Treatment
Currently, two basic surgical techniques are used: the hemi-Fontan operation or bidirectional Glenn anastomosis. The energy loss in the lateral intra-atrial tunnel produced after the previous hemi-Fontan operation is significantly lower compared to the energy dissipation in the tunnel produced after Glenns two-way operation. More physiological distribution of blood to both lungs after the hemi-Fontan surgery has been also noted . The second stage of treatment after previous treatment hybridization is a more complicated and longer operation. It is a combination of the Norwood operation and hemi-Fontan operation and in some centers also serves as preparation for performing the third stage of invasive cardiological defect treatment .
Traditionally, the bidirectional Glenn anastomosis was situated between the superior vena cava and the pulmonary arteries. The inferior vena cava to pulmonary artery anastomosis was abandoned in the animal lab by Dr. Glenn after repeated failures in his animal models. In patients with unfavorable upper body systemic venous anatomy, the superior vena cava –pulmonary artery connection is suboptimal or not feasible, and an alternative is needed to unload the heart. As it was demonstrated by studies, this subset of patients may benefit from the primary inferior vena cava -pulmonary artery connection, the Southern Glenn, which we have performed successfully in two patients .
